Every pill you take has a digital fingerprint
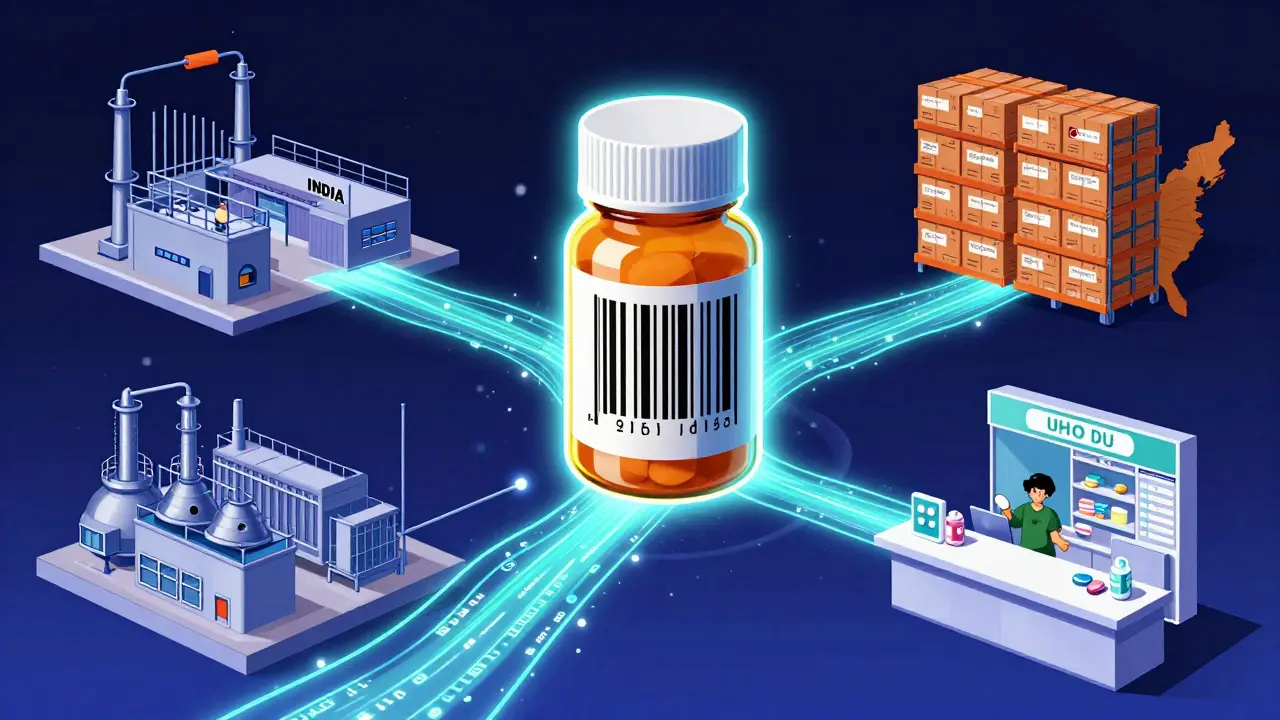
When you pick up a prescription at the pharmacy, you probably don’t think about how many hands it passed through before reaching you. It traveled from a factory in India or Germany, through warehouses in Ohio or Texas, maybe a distributor in Pennsylvania, and finally to your local pharmacy. And somewhere along that path, someone could have swapped it for a fake. Counterfeit drugs don’t just look wrong-they can be deadly. That’s why the U.S. pharmaceutical supply chain now runs on something no one sees: a digital security system built to track every single package. It’s not science fiction. It’s law. And it’s working.
How the system was built-step by step
The Drug Supply Chain Security Act (DSCSA) didn’t come out of nowhere. It was signed into law in 2013 after years of warnings. In 2012, a contaminated steroid injection killed 64 people and sickened over 750 across 20 states. That outbreak traced back to a rogue compounding pharmacy. The public demanded answers. Congress responded with DSCSA, a 14-year plan to lock down the drug supply chain. It wasn’t rushed. It was rolled out in phases so companies could adapt.
By 2017, every prescription drug package had to carry a unique identifier-a 2D barcode with the drug’s name, batch number, expiration date, and a one-of-a-kind serial number. That’s over 1.2 million new barcodes generated every day across the U.S. By 2023, all trading partners-manufacturers, wholesalers, pharmacies-had to exchange that data electronically. No more paper. No more hand-written logs. Everything had to be digital, traceable, and verifiable.
The goal? If a fake drug shows up, you can find it in minutes, not weeks. If a batch is recalled, you know exactly which stores got it. That’s what happened in 2022 during the infant formula crisis. When contaminated product was found, the system pinpointed affected lots and pulled them off shelves in under 72 hours. Before DSCSA, that would’ve taken two weeks.
The tech behind the safety net
At the heart of this system is something called EPCIS-Electronic Product Code Information Services. It’s a global standard created by GS1, the same group that made barcodes for groceries. EPCIS lets every company in the chain share data in real time. When a drug leaves the factory, the manufacturer logs its serial number. When it arrives at a warehouse, the warehouse scans it and logs its location. When it gets to the pharmacy, the pharmacist scans it again before handing it to you.
Each scan creates a digital trail. If someone tries to slip in a fake pill, the system flags it. The pharmacy’s system checks the serial number against the manufacturer’s database. If it doesn’t match, the drug is quarantined. In 2022, this process stopped over 12,000 suspect products from reaching patients.
It’s not just barcodes. Companies now use cybersecurity standards like HITRUST CSF v11.2 to protect data. Warehouse systems scan packages at 1,200 per minute with near-zero error rates. Even the smallest pharmacies now use handheld scanners linked to cloud systems. The tech is simple, but the scale is massive.
Who’s responsible-and who’s falling behind
DSCSA applies to everyone: manufacturers, repackagers, wholesalers, distributors, and dispensers (that’s pharmacies and hospitals). By December 2023, 92% of manufacturers and 87% of wholesalers were fully compliant. Hospitals were at 83%. But independent pharmacies? Only 76% made it.
Why? Cost. A small pharmacy with 10 employees might spend $18,500 a year just on software licenses and barcode scanners. That’s 3.2% of their net profit. Many didn’t have the IT staff to handle the upgrade. Some still use old systems from the 1990s. The FDA says 63% of small pharmacies struggled with the 2023 electronic data exchange deadline.
Even big companies aren’t perfect. A 2022 FDA audit found only 47% of wholesale distributors were doing required checks on their trading partners. That’s a loophole. If you don’t verify who you’re buying from, a fake drug can slip in.

What’s different in Europe-and why it matters
The U.S. isn’t alone. The European Union has its own system: the Falsified Medicines Directive (FMD). It’s stricter in some ways. Every prescription drug there must have a tamper-proof seal and a unique code that’s checked at the pharmacy counter. The code is then “decommissioned”-meaning it’s marked as used, so it can’t be reused on a fake.
The EU uses a centralized database. All pharmacies connect to a national system that checks codes against a single, EU-wide registry. The U.S. system is decentralized-each company keeps its own data and shares it directly with others. Neither is perfect. The EU system is easier to audit. The U.S. system is more flexible and faster to scale.
But here’s the problem: a drug made in the U.S. and shipped to Germany has to meet both systems. That means two sets of barcodes, two sets of software, two sets of training. Global pharmaceutical companies pay 22% more to comply with all these rules than U.S.-only companies do.
Real wins-and real gaps
The results speak for themselves. Since DSCSA started, counterfeit drug seizures dropped by 63%. In 2014, the FDA seized 1,103 fake drug packages. In 2022, that number was down to 412. That’s real lives saved.
During the pandemic, the system helped track over 98% of COVID-19 vaccine shipments. If a vial was damaged or expired, it was caught before it got to a clinic. That level of control was unimaginable a decade ago.
But there are still blind spots. Repackaged drugs-like when a hospital breaks down a bottle of 100 pills into smaller doses-are a headache. The original barcode is destroyed, and the new label might not carry the same level of security. And international borders are still vulnerable. A drug might be perfectly legal in the U.S., but if it’s shipped through a country with weak tracking, it could be swapped.
And then there’s cybersecurity. In early 2023, a cyberattack on Change Healthcare disrupted DSCSA verification for 72 hours. Over a third of U.S. pharmacies couldn’t check if drugs were real. That’s a terrifying gap.
What’s next? The road to 2027
The final deadline is November 2027. By then, every single transaction in the U.S. drug supply chain must be electronic, interoperable, and secure. No paper. No manual entries. All systems must talk to each other without glitches.
Right now, 78% of companies still use older XML formats for data sharing. By 2025, they all have to switch to JSON-a faster, simpler format. The FDA is running pilot programs with major companies to test full interoperability. Early results show 99.9% accuracy in data exchange.
Some companies are already looking beyond compliance. A third of top pharmaceutical firms are testing blockchain to make the chain even more tamper-proof. Others are using AI to spot unusual patterns-like a drug being ordered in bulk from a new supplier with no history. That’s the future: not just tracking, but predicting.
By 2030, experts predict the system will evolve into a predictive analytics platform. It won’t just stop fake drugs-it’ll prevent them from ever being made. And it could save the industry $8.7 billion a year in waste, recalls, and fraud.
What you can do
You don’t need to understand EPCIS or GS1 barcodes. But you can be smarter about your meds. Always check the packaging. Does it look off? Is the label blurry? Are the pills a different color than usual? If something feels wrong, don’t take it. Talk to your pharmacist. Report it to the FDA’s MedWatch program.
Counterfeit drugs are rare in the U.S. today-thanks to this system. But vigilance still matters. The tech is strong. But the human eye is still the last line of defense.
Frequently Asked Questions
What is DSCSA and why does it matter?
DSCSA stands for the Drug Supply Chain Security Act. It’s a U.S. law passed in 2013 to prevent counterfeit, stolen, or otherwise harmful drugs from entering the supply chain. It requires every prescription drug package to have a unique serial number and mandates electronic tracking from manufacturer to pharmacy. This system has cut counterfeit drug seizures by 63% since 2015 and allows for rapid recalls when needed.
How do I know if my medicine is real?
You can’t scan the barcode yourself, but you can look for signs. Check the packaging for misspellings, poor print quality, or mismatched colors. Compare the pills to pictures on the manufacturer’s website. If the bottle looks tampered with or the pills smell odd, don’t take them. Ask your pharmacist to verify the drug’s source. Most pharmacies now scan every prescription before dispensing, so your medicine has already been checked.
Are online pharmacies safe?
Only if they’re verified. Look for the VIPPS seal (Verified Internet Pharmacy Practice Sites) from the National Association of Boards of Pharmacy. Avoid websites that sell prescription drugs without a valid prescription, or that offer drugs at prices that seem too good to be true. The DSCSA only applies to U.S.-licensed pharmacies. Drugs shipped from overseas aren’t covered by this system and carry a much higher risk of being fake.
Why do some pharmacies still struggle with compliance?
Small, independent pharmacies often lack the budget and IT staff to upgrade legacy systems. Installing new scanners, software, and cybersecurity tools can cost over $18,000 a year. Many operate on thin margins. While big chains had resources to adapt, smaller ones were left behind. The FDA and pharmacy associations are offering grants and training, but full compliance by 2027 remains a challenge for these businesses.
What happens if a fake drug gets through?
If a pharmacy scans a package and the system flags it as suspicious, the drug is immediately quarantined. The pharmacy must report it to the manufacturer and the FDA within 24 hours. The manufacturer investigates the serial number, checks production logs, and determines if it’s counterfeit. If confirmed, the FDA issues a recall, and all affected batches are pulled from the market. In 2022, over 12,000 suspect products were stopped before reaching patients thanks to this process.
What’s next for you
If you’re a patient, stay alert. If you’re a pharmacist or distributor, keep training. If you’re in the industry, keep investing. The system isn’t perfect, but it’s the most advanced drug safety network ever built. And it’s still getting smarter. The next decade won’t just be about stopping fakes-it’ll be about preventing them before they’re made.

Chris & Kara Cutler
February 2, 2026 AT 00:47OMG this is insane 😱 I had no idea my insulin had a digital fingerprint! Like, my phone can track my steps but my medicine has a whole blockchain trail? 🤯 We’re living in the future and I didn’t even get a memo.
Rachel Liew
February 3, 2026 AT 12:31i just want to say thank you to all the pharmacists who scan every single bottle. i have a kid with allergies and knowing that the meds are real makes me sleep better. even if the system is complicated, the fact that it works? that’s everything. ❤️
Lisa Rodriguez
February 4, 2026 AT 19:00Big shoutout to the small pharmacies trying to keep up with all this tech - i know it’s expensive and confusing but you’re doing the work that saves lives. I’ve seen my local pharmacy upgrade their scanners and honestly? It’s kinda beautiful to see a mom-and-pop shop fighting to stay safe. We need more of this, not less. 🙌
Also, the EU vs US thing is wild. I’ve had meds from both sides and honestly, the EU seal feels more like a spy movie. But the US system just… works without me having to think about it. That’s the win.
Lilliana Lowe
February 6, 2026 AT 14:16While it’s commendable that the DSCSA has improved supply chain integrity, one must acknowledge the inherent structural inefficiencies of a decentralized, proprietary data architecture. The absence of a unified, cryptographically verifiable ledger renders the entire system vulnerable to sybil attacks and interoperability failures - particularly in light of the Change Healthcare breach. One cannot rely on XML-to-JSON migration as a panacea when the foundational trust model remains fragmented. The EU’s centralized registry, despite its bureaucratic overhead, is architecturally superior. This is not opinion - it is systems theory.
Melissa Melville
February 7, 2026 AT 20:05So let me get this straight - we spent 10 years and billions to make sure my blood pressure pill isn’t a sugar cube with a fake label… but my Amazon package still shows up in a box that says ‘Fragile: Glass’ with a picture of a cat? 🤔
At least my meds have a better security team than my Netflix account.
Deep Rank
February 8, 2026 AT 10:22okay but honestly this whole system is just a distraction from the real problem - why are drugs so expensive in the first place? if you can track every pill from india to my pharmacy, why can’t you track why it costs $400? this tech is cool but it’s like putting a fancy lock on a house that’s built on quicksand. also i read somewhere that 70% of fake pills come from china and the u.s. still imports them? so what’s the point? we’re just making the system look good while the real danger is still coming in the mail. and don’t even get me started on how the big pharma companies are probably using this to push out small labs that make generics. this isn’t safety - it’s consolidation with extra steps.
Bryan Coleman
February 9, 2026 AT 14:16My local pharmacy had a guy come in last month trying to return a bottle of metformin. Scanner flagged it - serial number didn’t match. Turned out he bought it off some guy on Facebook for $10. The pharmacy held onto it, reported it, and now it’s in an FDA evidence locker. I just got my refill today - scanned it, everything green. Feels good to know the system actually works. No drama. Just quiet, reliable tech doing its job.
Donna Macaranas
February 11, 2026 AT 10:09I love that this system exists, but I also feel bad for the little pharmacies. My aunt works at one in rural Kansas - she’s 62, learned how to use a computer at 50, and now she’s supposed to manage cloud-based barcode logs? She’s doing her best. I wish there was more support for them. Not just rules - actual help. Maybe a tech volunteer corps? Like AmeriCorps but for pharmacy scanners?
Nidhi Rajpara
February 12, 2026 AT 08:46As a pharmaceutical supply chain analyst based in India, I must emphasize that while the DSCSA is robust, it fails to account for the global reality of drug manufacturing. Over 70% of APIs originate in India and China, yet these countries are not bound by the same traceability mandates. The U.S. system is a fortress - but the gates are wide open for foreign-manufactured inputs. Until global harmonization occurs, this is merely a domestic illusion of security. Compliance is not safety - it is paperwork with a barcode.